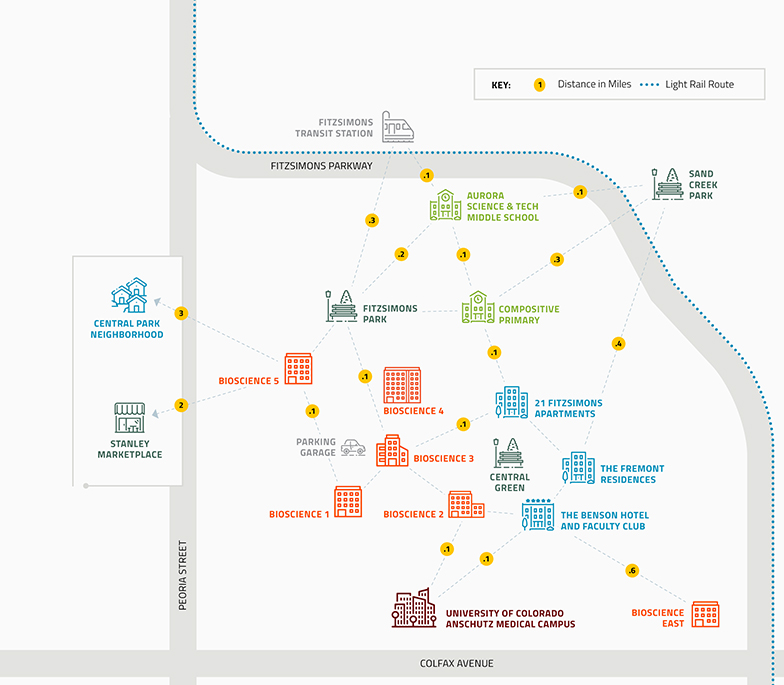
Vona Oncology:
Providing New Hope for Patients with Aggressive Cancers
What the Company Does
Vona Oncology is an early clinical stage bio-pharmaceutical company developing targeted therapeutics to treat aggressive cancers such as triple-negative breast cancer (TNBC), ovarian cancer, and melanoma. Vona Oncology has an innovative, novel small molecule that is very efficacious with little toxicity to improve standard of care.
Current Landscape
About 20 percent of women diagnosed with breast cancer are triple negative, which means therapy options with targeted drugs are very limited. TNBC is often diagnosed in younger women, at very advanced stages with high likelihood of relapse and metastasis. And, for unknown reasons, it’s often diagnosed in African American and Hispanic women, showing a significant racial disparity in this disease.
Chemo, radiation, and select targeted drugs are the current standard of care for patients with TNBC. Certain immunotherapies are approved in combination with chemo but patients are restricted from using these drugs unless they have specific tumors, which applies to a fraction of the people in need. Competitors include large pharmaceutical companies such as Roche, Bristol-Myers Squibb and AstraZeneca, and Gilead. Gilead’s FDA-approved drug Trodelvy is available for patients that did not respond to at least two prior treatments. However, it is infused on a once-weekly basis on a 21-day treatment cycle, and treatment continues until intolerable toxicity.
There continues to be a need for more tolerable agents such as Vona Oncology’s VDX-431. A biomarker-guided and cancer-specific drug, with the goal of minimal side effects.
Company Birth Story
Oncology is fundamental clinical challenge with significant unmet needs for different types of cancers including rare cancers. Jim Lambert, Ph.D., has over 30 years of experience in cancer research and discovered the unique anti-tumor properties of Vona Oncology’s novel small molecule in his lab at the University of Colorado. With his team, he identified a large opportunity to improve standard of care and started Vona Oncology at Fitzsimons Innovation Community in Colorado to accelerate pre-clinical studies, Investigation New Drug (IND) approval, and build a pathway for investigation of the drug in human clinical trials.
Solution
Vona Oncology is developing novel, first-in-class small molecules for aggressive and rare cancers where there remains urgent unmet needs for new therapies. The drug they are developing, VDX-431, is a small molecule, well-tolerated drug that will establish a novel class of targeted therapy for treatment of TNBC. Studies have provided proof-of-concept that VDX-431 is an efficacious therapeutic for TNBC in pre-clinical cell and animal models. Interestingly, it does not kill normal cells. The more aggressive the cancer, the better the drug works.
The company’s immediate objective is the completion of IND-enabling studies of VDX-431 and demonstration of early clinical phase efficacy in the treatment of TNBC. The long-term vision is to grow Vona Oncology into a cutting-edge drug discovery and development company that can turn novel biological insights in cancer biology into actionable clinical treatments.
Team Culture
A diverse and talented team of scientists, clinicians, business executives and consultants lead to a dynamic sharing of ideas and approaches to further the company’s mission.
Founder Quote
“Vona is the Icelandic word for “hope”. Our mission is to provide new hope for patients with aggressive cancers where novel therapies are needed.”
Jim Lambert, Ph.D., President, Vona Oncology
Learn More
Website: vonaoncology.com